|
This
story, concerned as it is with life-saving drugs, brings
into stark relief the nature of the international pharmaceutical
industry and the implications of the TRIPS agreement
for basic public health. Conversely, the multinational
pharmaceutical companies argue that without intellectual-property
protection they would have no incentive to invest the
millions required to discover and develop new drugs.
The irony in this case is that the costs of developing
the two chemicals (AZT and 3TC) used in Glaxo's Combivir
were actually borne by the public sector - through work
done earlier by researchers funded by the US Government's
National Institute of Health. Glaxo purchased the drug
before its efficacy in AIDS treatment became obvious,
and once again relied on public research to establish
this efficacy. However, once it was known, the company
lost no time in taking out a patent on this drug and
reaping monopoly profits from its sale.
Pharmaceutical markets differ from markets for most
other commodities, since drugs are a rather special
commodity. Private drug markets typically suffer from
a number of forms of market failure, including (a) informational
imbalances; (b) monopoly and lack of competition created
by patent protection, brand loyalty and market segmentation;
(c) externalities in the form of social benefits of
drug consumption. In addition, of course, there are
obvious welfare implications, since unregulated drug
markets tend to create substantial inequity, particularly
in terms of access to drugs.
For these reasons, there have been major concerns about
the enforcement of the TRIPS agreement particularly
with reference to health conditions in developing countries.
Some of the most frequently expressed concerns have
included the following :
(i) Increased patent protection will lead to higher
drug prices, while the number of patented drugs of importance
from a public-health point of view will increase in
the coming years.
(ii) The access gap between developed and developing
countries, and between rich and poor in all countries,
will increase, especially as producers in developing
countries would have to wait for 20 years before they
can have access to innovations.
(iii) Enforcement of the WTO regulations will have an
effect on local manufacturing capacity and remove a
source of generic innovative quality drugs on which
the poorer countries depend.
(iv) While technology transfer will actually be discouraged,
there are no incentives or provisions to ensure that
increased revenues will go towards the development of
essential medical technologies.
Further, while the TRIPS agreement still leaves some
loopholes for developing countries in the form of compulsory
licensing and parallel imports, it is increasingly difficult
to choose these options. This is because the industrial
countries - and the US in particular - have been pressurising
several developing countries to implement patent and
intellectual property legislation that is more restrictive
than the minimum requirements of the TRIPS Agreement.
These possibilities become more disturbing given the
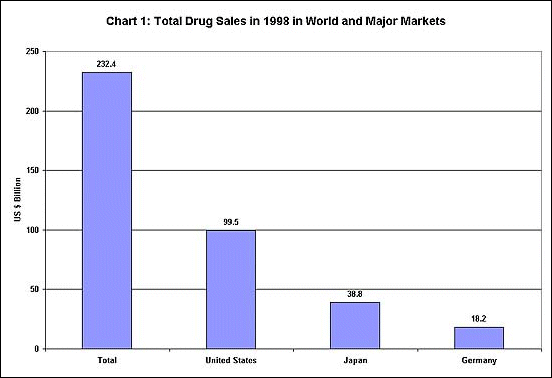
nature of the international drug market. As Chart 1
shows, the world market for drugs is a huge one, but
it is dominated by only 3 countries - the United States,
Japan and Germany - which make up more than two-thirds
of total sales. Chart 2 shows an even more unequal distribution
: only 15 per cent of the world' population accounts
for 86 per cent of drug spending, while the remaining
85 per cent of the world's population get only 14 per
cent share.
Chart
1 >> Click
to Enlarge
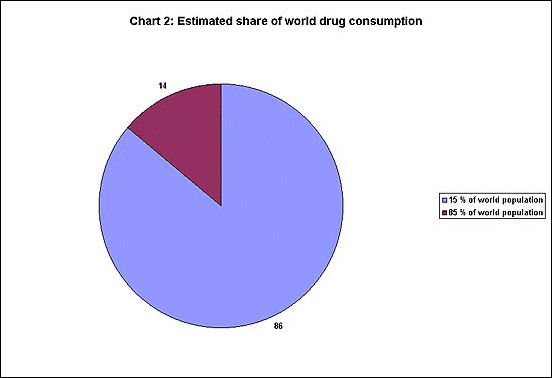
Chart 2 >> Click
to Enlarge
|

